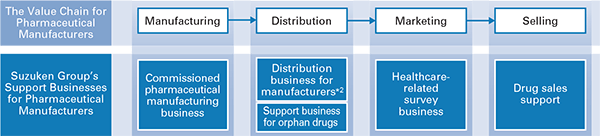
Healthcare-Related Services Business
We provide manufacturer support services such as contract distribution for manufacturers and contract distribution of orphan drugs to meet diverse needs of pharmaceutical manufacturers in Japan and overseas, and digital healthcare services to solve issues in the healthcare field.
Opportunities and risks
Opportunities
- Expansion of the specialty drug and biopharmaceutical markets
- Foreign pharmaceutical companies entering the Japanese market
- Promotion of comprehensive community care system construction
- Increase in elderly population in large metropolitan areas
- Expansion of long-term care service scope due to aging population
- Advance of digitalization in the medical and healthcare fields
- Progress in personalized medicine
- Diversifying needs for in-home medical treatment and nursing care Increase in digital health services
- Healthcare data linkage and use
Risks
- Mandatory compliance with GDP in pharmaceutical distribution
- The 2024 problem in logistics
- Increasingly severe competition due to entrants from other industries
- Revision of Guidelines for the Improvement of Commercial Transaction Practices
Suzuken Group strengths
Know-how and track record in manufacturer distribution and specialty drug distribution
- Greater shipping efficiency and optimization of distribution stock through shared distribution
- GDP-compliant quality management and nationwide shipping and delivery network
- Distribution platform for regenerative medicine products
- Development and placement of GDP specialists
- Contract manufacturer distribution service (As of March 31, 2024): 48 companies
- Contract specialty drug distribution service (As of March 31, 2024): 60 items, 34 companies
Customer support services utilizing digital means
- Development and rollout of the COLLABO Portal, a comprehensive portal site for medical and nursing care providers Registered users (as of March 31, 2024):Approx. 160,000 IDs
Locations of the manufacturer distribution service (As of March 31, 2024)
- Manufacturer distribution centers:11
- ISO 9001:2015 certified locations:Koga, Sugito, Higashi Nihon, Tsukuba, Kobe, Amagasaki, Nishi Nihon, and Rokko manufacturer distribution centers, Chuounyu headquarters, and Iwatsuki office
Main initiatives
Manufacturer Support Services Business
Providing a variety of services to pharmaceutical manufacturers
We contribute to pharmaceutical manufacturers' cost reductions by contracting pharmaceutical manufacturing, operating manufacturer distribution centers and contracting wholesaler distribution. Further, we regard it as our social mission to contribute to the distribution of orphan drugs※1, ensuring that necessary drugs are delivered even if there is only one patient who suffers from an illness.
-
※1Drugs with high medical needs but required by only a small number of patients
-
※2Distribution for manufacturers: In the above diagram, "procurement distribution" means transportation of pharmaceutical raw materials to the manufacturer's factory; "production distribution" means transportation of pharmaceuticals from the manufacturer's factory to its warehouses; and "sales distribution" means transportation of pharmaceuticals from the manufacturer's warehouses to our wholesale distribution centers.
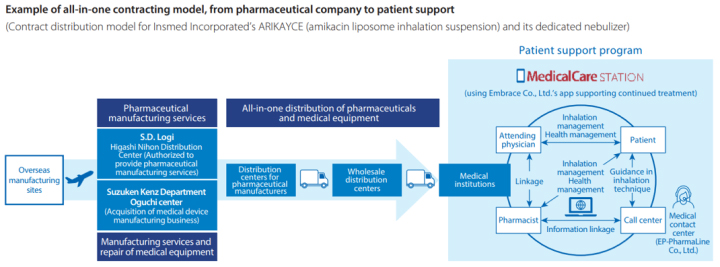
Functional expansion of our all-in-one contracting model
In recent years, the number of foreign pharmaceutical companies aiming to enter the Japanese market has been increasing, but many have no manufacturing plants, distribution centers, or a distribution network. To meet these needs, in April 2021, we entered into a collaboration with Bushu Pharmaceuticals Ltd., and combining the capabilities of our two companies has allowed us to undertake the all-in-one contract provision of specialty drug services ranging from marketing authorization holders’ consulting to import, inspection, manufacturing, distribution, post-marketing surveillance, and patient support. Additionally, we have a manufacturing operations function at a distribution center owned by Chuounyu Co., Ltd., a member of the Suzuken Group. We have absorbed EP-PharmaLine Co., Ltd., a subsidiary of EPS Holdings, Inc., with whom we began a capital and business alliance in 2016. And we integrated EP-PharmaLine’s medical contact center functions and BPO functions, for example, with the Suzuken Group’s functions to build a platform that meets pharmaceutical companies’ needs all in one stop.
In January 2023, in partnership with pharmaceutical companies, we started the industry’s first joint shipping, in compliance with GDP guidelines. By improving the efficiency of distribution from pharmaceutical companies to wholesale distribution centers, we are also helping to reduce the environmental burden.
Realizing Quality Management in Line with Global PIC/S GDP Standards
In our contract distribution business for pharmaceutical manufacturers, which the Suzuken Group began providing in 2005, we practice strict temperature and quality control in conformance with GMP※3 standards. At our manufacturer distribution centers, we gained GMP-compliant ISO 9001 certifications in 2008, have updated those certifications to the 2015 version, and provide quality control in conformance with the PIC/S※4 GDP※5 global standards. With the start of operations at CHUOUNYU CO., LTD.’s Medical Terminal (photo on page 42) in October 2018, more efficient joint pharmaceutical distribution is now being provided in the Kanto Area. In addition, efforts are being made to expand the area in which we are able to offer our Direct Cool※6 refrigerated distribution service for pharmaceuticals.


-
※3GMP (Good Manufacturing Practice): Manufacturing and quality management guidelines for pharmaceuticals
-
※4PIC/S: A combination of abbreviations for the Pharmaceutical Inspection Convention (PIC) and the Pharmaceutical Inspection Co-operation Scheme (PICS), which are two international cooperation organizations that aim to improve cooperation among governments and inspection authorities in the areas of GMP and GDP
-
※5GDP (Good Distribution Practice): Quality management guidelines for the transportation and storage of pharmaceuticals
-
※6Direct Cool: Temperature controlled service in which pharmaceuticals (2~8°C) are shipped from Chuounyu Medical Terminals throughout the country directly to pharmaceutical wholesale distribution centers
Strengthening of distribution platform for CGT products
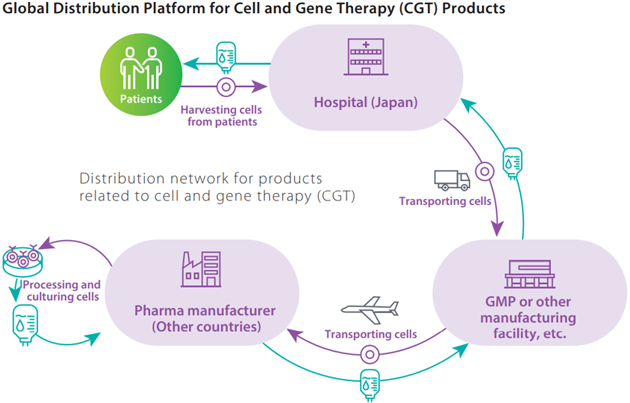
In 2016, we initiated the distribution of clinical trial products for cell and gene therapy (CGT), and we collaborated in 2018 with World Courier, a part of the U.S.-based Cencora (former AmerisourceBergen) drug wholesale group, to create a global network for CGT products. We are expanding our contract distribution services for pharmaceutical companies that aim to enter the Japanese market and launch new products, such as Novartis Pharma K.K.’s Kymriah, Japan’s first CAR-T cells medication, and ZOLGENSMA IV infusion, a gene therapy product for treating spinal muscular atrophy, Together with SanBio, Inc., we jointly developed the R-SAT support system for CGT products distribution management and drug administration scheduling, and we obtained a patent for it in April 2022. In addition, through initiatives such as obtaining manufacturing authorization for storage, labelling, and decision-making for product shipping, a first for a pharmaceutical wholesaler, we are expanding our services for responding to the sophisticated, diversifying needs of pharmaceutical companies.
Digital Services Business
Creation of digital services through collaboration with health-tech companies
Since 2019, the Suzuken Group has been actively advancing partnerships with health-tech companies. After entry into capital and business alliances with Dr. JOY Co., Ltd., Which provides solutions to improve efficiency in clinical environment and with Welby Inc., a leading company in personal health record (PHR) services, we formed a similar alliance with Ubie, Inc. in April 2020 and embarked on the joint promotion of the Ubie for Hospitals medical interview service for medical institutions.
Creation of digital services through collaboration with health-tech companies
Since 2019, the Suzuken Group has been actively advancing partnerships with health-tech companies. After entry into capital and business alliances with Dr. JOY Co., Ltd., which offers efficiency solutions for healthcare settings, and with Welby Inc., a leading company in personal health record (PHR) services, we formed a similar alliance with Ubie, Inc. in April 2020 and embarked on the joint promotion of the Ubie for Hospitals medical interview service for medical institutions. In November 2020, we entered into a capital and business alliance with Doctors Inc., a company building and promoting a healthcare DX platform from the perspective of frontline healthcare professionals. April 2021 saw the addition of Embrace Co., Ltd., a capital and business partner since February 2020, as a Suzuken subsidiary, and now we are developing business based on a platform applying Medical Care Station (MCS). In November 2021, we entered into a capital and business alliance with FRONTEO, Inc., which operates a data analysis business using artificial intelligence (AI). We are conducting distribution-related activities on an exclusive basis for the conversational dementia diagnosis support AI program that FRONTEO provides, and we aim to establish a system to quickly penetrate and expand the market.
In February 2022, we began building a new added value service for the Cubixx System with SUSMED Inc., with whom we formed a capital and business alliance in May 2020. We will leverage that company’s digital healthcare platform, which consists of blockchain technology for preventing data falsification and identity theft, its AI-based automated analysis systems, and other technologies, to enable communication activities and the optimization of market inventory management.
Together with a variety of companies, we will launch digitalization efforts to solve the problems that the industry is facing and to build a safe and secure healthcare platform, thereby contributing to patients and to community healthcare and the healthcare professionals that support it.
Establishment of new companies to promote digital business
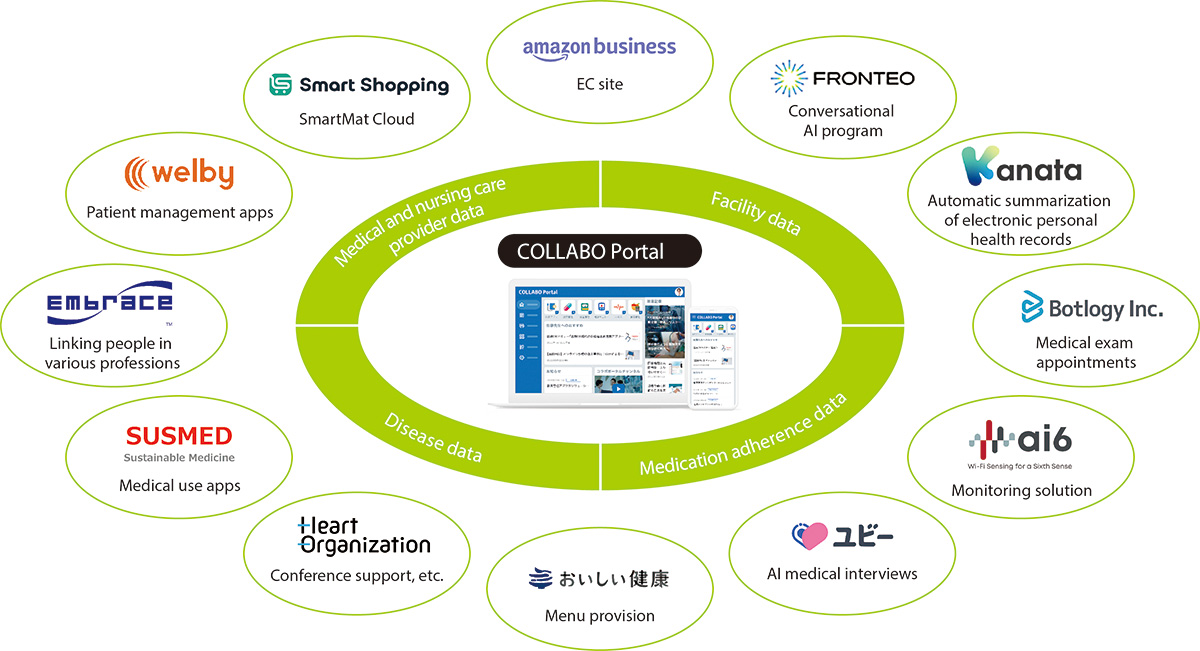
With the increase in digital healthcare services, we believe that a new “digital wholesaler” function is necessary to manage the distribution of healthcare apps, programs, and other digital materials. Making the best use of our Group strengths as “connecting capability” and “spreading capability,” we are now developing a comprehensive, all-in-one portal site that seamlessly “connect”s doctors, pharmaceutical companies, and patients with a variety of digital healthcare services from both Japan and overseas which we will “spread” on the platform. We will ensure that this site contributes to customers and community-based healthcare and provides optimal solutions to pharmaceutical companies.
In addition, we are looking into establishing a Health-tech Collaboration Council (tentative name) with the goal of ensuring that a safe, secure, and correct concept of DX penetrates and takes root, one that creates a level of security and compliance that serves as the industry standard. Furthermore, we set up a Corporate Venture Capital (CVC) fund to shift investment in health-tech companies into full swing in April 2022. We will partner with a variety of health-tech companies that have leading-edge technology, business models, and ideas, to create new solutions.
Building a digital platform
Health-tech companies produce outstanding products and technologies from scratch, but they do not have the resources and channels for popularizing them. For its part, the Suzuken Group is rolling out a digital health business that utilizes our network of medical institutions and pharmacies and other customers that we have cultivated thus far to apply health-tech companies’ technology in society, increasing that value 100-fold or 1,000-fold. We aim to create new added value by connecting individual services and data, with features such as a system that allows customers to access a variety of services and obtain diverse information using a single log-in, and a mechanism that organically interlocks data that can be utilized to create new solutions.
To construct the digital platform that will realize these mechanisms, in March 2022, we established Collabo CREATE CO. LTD., which will conduct the planning and proposals for the platform, and Collabo PLACE CO. LTD., which will manage its development, construction, and operations. And in April 2023, we began rolling out COLLABO Portal, a comprehensive portal site for medical and nursing care providers. This portal will fulfill the role of connecting our partner health-tech companies with patients, medical and nursing care providers, pharmaceutical companies, local governments, and the Suzuken Group. We have begun offering services nationwide and already have over 160,000 IDs registered.
We also began rental services in June 2023 for COLLABO Mobile, a communications device that will enable medical and nursing care providers to access digital health services all in one place when needed. COLLABO Portal’s functions are installed as standard on COLLABO Mobile, and it can be used not only in facilities but also at patients’ homes and other home healthcare and nursing care sites in the community.
Popularization and expansion of services in preparation for digital business rollout
We are aiming to realize profitability for digital businesses using COLLABO Portal in fiscal 2025. But first, we plan to focus on popularizing and expanding the portal and building data use and application schemes, thereby increasing the portal’s value.
Centering on COLLABO Portal, we will roll out a wide range of services that support medical and nursing care providers. Utilizing the services-related information and patients’ ailments and medication data that are accumulated there, we aim to create services that meet diversifying needs and to realize a platform that is appealing to medical and nursing care providers.
Related group companies and affiliates
Subsidiaries
Healthcare-Related Services Business
- CHUOUNYU CO., LTD.
- Net Hospital, Inc.
- P.J.D. Network
- JIT Co.,Ltd.
- S.D. Collabo Co., Ltd.
- Suzuken Business Associe Co., Ltd.
- Embrace Co., Ltd.
- Galenus Co., Ltd.
- Life Support Co.,Ltd.
- Suzuken JoinUs Co., Ltd.
- Collabo Square Co., Ltd.
- Suzuken Investment Co., Ltd.
- Kyuyaku Service Limited
Associated Companies
Healthcare-Related Services Business
- EP-PharmaLine Co., Ltd.
- Welby Inc.
