Specialty Drug Contract Distribution Business
This refers to a business where we contract distribution of pharmaceutical products including orphan drugs that requires stricter quality control and distribution management compared to the typical distribution channels, from manufacturers. With the healthcare distribution platform as its foundation, the Suzuken Group has the industry-leading track record in the contract distribution of specialty drugs, which require strict temperature, inventory, and security management.
Since our entry into the manufacturer distribution business, we have contributed to the improvement of the quality of pharmaceutical distribution and the reduction of social costs by advancing our services and working on further expansion of business.
Opportunities and risks
Opportunities
- Expansion of the specialty drug and biopharmaceutical markets
Risks
- Mandatory compliance with GDP in pharmaceutical distribution
Suzuken Group strengths
Know-how and track record in specialty drug distribution
- Greater shipping efficiency and optimization of distribution stock through shared distribution
- GDP-compliant quality management and nationwide shipping and delivery network
- Distribution platform for regenerative medicine products
- Development and placement of GDP specialists
- Contract specialty drug distribution service (As of March 31, 2025): Products share ≧ 50 %, 70 items, 39 companies
Main initiatives
Initiated contract provision of manufacturer distribution as a response to the outsourcing needs of pharmaceutical companies
In 2005, the Suzuken Group became the first pharmaceutical wholesaler to offer contract distribution for pharmaceutical manufacturers. Offering greater delivery efficiency and distribution inventory optimization by responding to pharmaceutical company outsourcing needs while continuing to provide customized distribution services to pharmaceutical company customers, we are helping to reduce social costs. The Suzuken Group currently operates 11 manufacturer distribution centers and six transportation terminals. Since 2008, eight distribution centers have obtained GMP※1 -compliant ISO9001 certifications. They later upgraded their certifications to the 2015 version, conform to the PIC/S※2 GDP※3 global standards, abide by a new set of operating standards known as the Good Distribution Practice Guidelines, and go to other lengths, as well, to achieve even better quality control. Applying know-how we developed in manufacturer distribution to our work in wholesale distribution, we have built a national distribution network that enables the provision of high distribution quality.
Being the industry’s first provider of contract manufacturing distribution, through our track record as the industry’s top contractor in specialty drug distribution we have been able to build up a corps of human resources that have a wealth of experience and know-how, and these resources are the greatest strength of the Suzuken Group.
Also, S.D.Logi, which is in charge of our logistics function, is strengthening our GDP efforts by merging people and systems, through initiatives such as developing employees internally qualified as GDP specialists and placing them in every prefecture in Japan, and by carrying out the practice, management, education, and audits of warehouse and delivery tasks in compliance with GDP guidelines.
-
※1GMP (Good Manufacturing Practice): Manufacturing and quality management guidelines for pharmaceuticals
-
※2PIC/S: A combination of abbreviations for the Pharmaceutical Inspection Convention (PIC) and the Pharmaceutical Inspection Co-operation Scheme (PICS), which are two international cooperation organizations that aim to improve cooperation among governments and inspection authorities in the areas of GMP and GDP
-
※3GDP (Good Distribution Practice): Quality management guidelines for the transportation and storage of pharmaceuticals
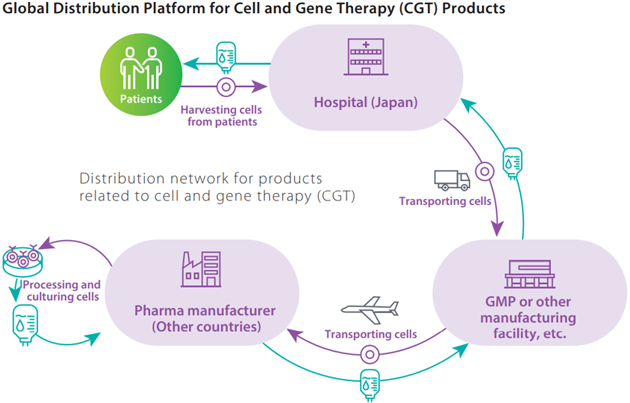
Providing distribution services in the fields of rare diseases and cell and gene therapy (CGT)
In 2012, we became the first pharmaceutical wholesaler to offer comprehensive support services in the field of rare diseases. In 2016, we added to these efforts by initiating the distribution of clinical trial products for cell and gene therapy (CGT). For these products, there are cases in which there is a need to collect tissue or cells in Japan, ship them overseas, where they are processed or cultivated, and then return them to Japan. To serve this need, we collaborated in 2018 with World Courier, a part of the U.S.-based AmerisourceBergen drug wholesale group, to create a global distribution platform for CGT products.
We are expanding our contract distribution services for pharmaceutical companies that aim to enter the Japanese market and launch new products, such as Novartis Pharma K.K.’s Kymriah, Japan’s first CAR-T cells medication, and ZOLGENSMA IV infusion, a gene therapy product for treating spinal muscular atrophy, Together with SanBio, Inc., we jointly developed the R-SAT support system for CGT products distribution management and drug administration scheduling, and we obtained a patent for it in April 2022. In addition, through initiatives such as obtaining manufacturing authorization for storage, labelling, and decision-making for product shipping, a first for a pharmaceutical wholesaler, we are expanding our services for responding to the sophisticated, diversifying needs of pharmaceutical companies.
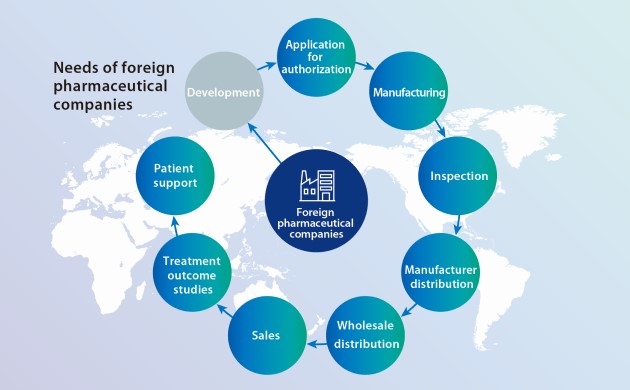
Responding to the needs of foreign companies newly entering Japan’s pharmaceutical market
In recent years, the number of foreign pharmaceutical companies aiming to enter the Japanese market has been increasing, but many have no manufacturing or distribution centers, or distribution network.
The Suzuken Group entered into a collaboration with Bushu Pharmaceuticals Ltd., in April 2021 and launched operations at the Greater Tokyo Distribution Center in April 2024. Combining the capabilities of our two companies will allow us to undertake the all-in-one contract provision of specialty drug services ranging from MAH※4 consulting, to import, inspection, manufacturing, distribution, post-marketing surveillance, and patient support. We believe that greater work efficiency and optimization of distribution stock achieved by condensing the supply chain and minimizing product movement will ultimately reduce pharmaceutical disposal losses.
As part of our collaboration, Bushu Pharmaceuticals will begin contract manufacturing within the Bushu Pharma Kazo Packaging Center, which will be established inside the Kazo Pharmaceuticals Joint Distribution Center of Group company Chuo Unyu Co., Ltd.. The company will also undertake comprehensive support activities including joint sales promotion activities in the U.S. to target pharmaceutical companies aiming to newly enter the Japanese market.
The Suzuken Group will continue to draw on its many years of accumulated know-how to strengthen our distribution platform for reliably delivering pharmaceuticals to patients eagerly anticipating new treatments, and create and provide services that respond to specialty drug distribution needs, as we strive to remain a company that plays an essential role for customers and society.
-
※4MAH(Marketing Authorization Holder) : Refers to a company engaged in pharmaceutical manufacturing and sales
Related contents
- S.D. Collabo Co., Ltd.
- Transformation to a health creation enterprise:Specialty Drug Contract Distribution Business
