Safe, secure, and reliable pharmaceutical distribution
Safe, secure, and reliable pharmaceutical distribution
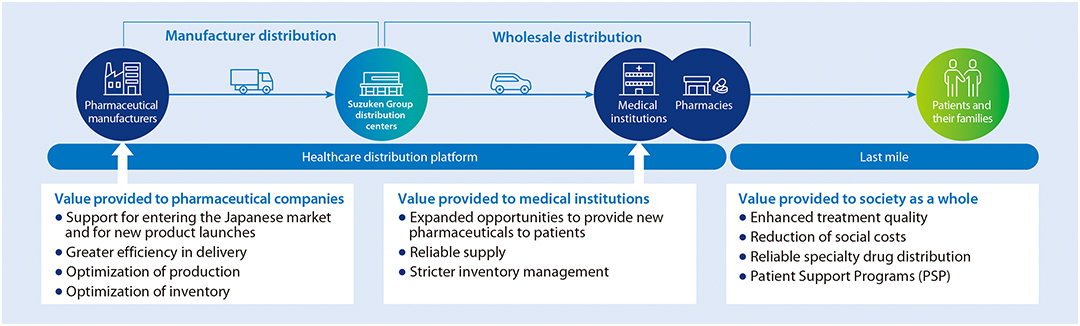
Our pharmaceutical distribution business has built a network that uses a traceability system to clearly identify distribution routes for all pharmaceuticals handled and, with strict quality control, reliably delivers required products at required times.
The Cubixx System, implemented in 2017, offers traceability for specialty drugs. In 2019, It was selected by the Japan Automatic Identification Systems Association (JAISA) to receive the Award for Excellence in the 21st Automatic Identification Systems Grand Prix,※1 in recognition of Cubixx’s economic efficiency, technological ingenuity, and convenience.
Moving forward, we aim to realize reliable supply and provide new added value by combining digital solutions to build a mechanism that considers the patient perspective and the sustainability perspective.
-
※1Automatic Identification Systems Grand Prix: With the goal of raising awareness about automatic identification technology and systems and promoting their development and spread, this program recognizes advanced and extremely effective technologies and systems relating to automatic identification
Quality control that meets global standards
S.D. Logi Co., Ltd., a provider of distribution services, acquired ISO 9001 certification in 2008 and has been performing quality control with consideration for GMP※2 in its manufacturer distribution services. The company later updated its certification to the 2015 version and now offers quality control in compliance with the PIC/S※3 and GDP※4 global standards. Currently, S.D. Logi is applying its accumulated expertise to the operation of wholesale distribution centers and business sites and focusing on further strengthening its all-in-one distribution quality control.
ISO 9001:2015 certification scope
Reliability Assurance Department, Wholesale Distribution Department, Miyagi Distribution Center, Osaka Office, Manufacturer Distribution Department, Kobe Distribution Center, Koga Distribution Center, Amagasaki Distribution Center, Sugito Distribution Center, Nishi Kobe Distribution Center, Nishi Nihon Distribution Center, Higashi Nihon Distribution Center, Rokko Distribution Center, and Tsukuba Distribution Center
-
※2GMP (Good Manufacturing Practice): Manufacturing and quality management guidelines for pharmaceuticals
-
※3PIC/S: A combination of abbreviations for the Pharmaceutical Inspection Convention (PIC) and the Pharmaceutical Inspection Co-operation Scheme (PICS), which are two international cooperation organizations that aim to improve cooperation among governments and inspection authorities in the areas of GMP and GDP
-
※4GDP (Good Distribution Practice): Quality management guidelines for the transportation and storage of pharmaceuticals
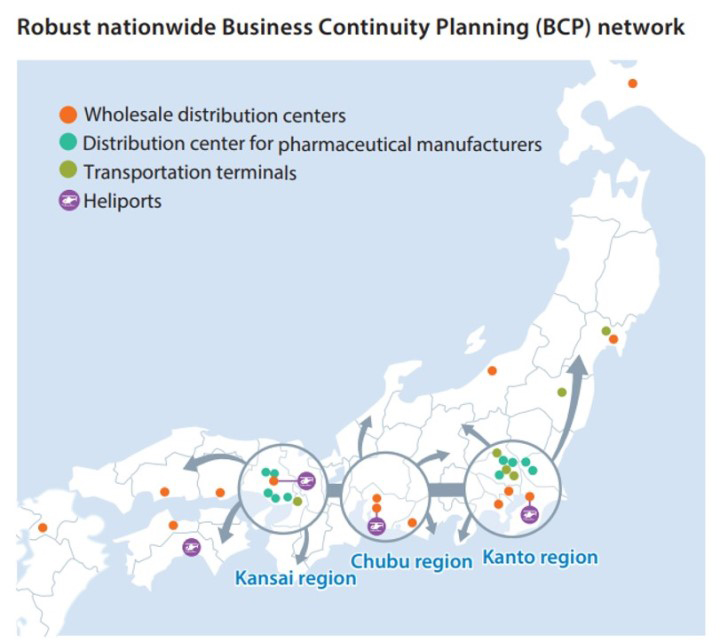
BCP initiative
Our manufacturer distribution and wholesale distribution businesses have worked together to construct a resilient nationwide distribution network with key facilities in Tokyo, Nagoya, and Osaka. In times of disaster, this distribution network will work with governmental authorities and pharmaceutical companies to swiftly deliver the pharmaceuticals needed by hospitals and other medical facilities in disaster zones
Key BCP facilities
| Facilities with on-site generators |
|
|---|---|
| Facilities storing diesel and gasoline |
|
| Satellite and priority phone system |
|
| Employee safety confirmation system |
|



