Specialty drug distribution
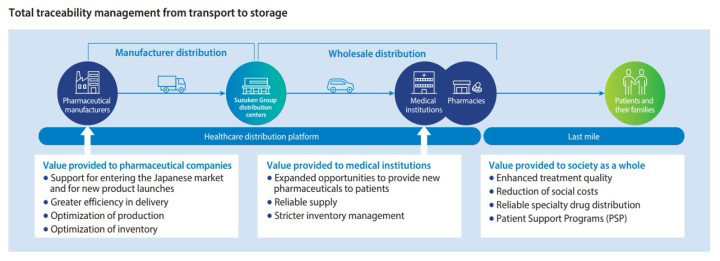
With the healthcare distribution platform as its foundation, the Suzuken Group has the industry-leading track record in the contract distribution of specialty drugs, which require strict temperature, inventory, and security management.
Since our entry into the manufacturer distribution business, we have contributed to the improvement of the quality of pharmaceutical distribution and the reduction of social costs by advancing our services and working on further expansion of business.
Initiated contract provision of manufacturer distribution as a response to the outsourcing needs of pharmaceutical companies
In 2005, the Suzuken Group became the first pharmaceutical wholesaler to offer contract distribution for pharmaceutical manufacturers. Offering greater delivery efficiency and distribution inventory optimization by responding to pharmaceutical company outsourcing needs while continuing to provide customized distribution services to pharmaceutical company customers, we are helping to reduce social costs. The Suzuken Group currently operates 11 manufacturer distribution centers and six transportation terminals. Since 2008, nine distribution centers have obtained GMP※1 -compliant ISO9001 certifications. They later upgraded their certifications to the 2015 version, conform to the PIC/S※2 GDP※3 global standards, abide by a new set of operating standards known as the Good Distribution Practice Guidelines, and go to other lengths, as well, to achieve even better quality control. Applying know-how we developed in manufacturer distribution to our work in wholesale distribution, we have built a national distribution network that enables the provision of high distribution quality.
Being the industry’s first provider of contract manufacturing distribution, through our track record as the industry’s top contractor in specialty drug distribution we have been able to build up a corps of human resources that have a wealth of experience and know-how, and these resources are the greatest strength of the Suzuken Group.
-
※1GMP (Good Manufacturing Practice): Manufacturing and quality management guidelines for pharmaceuticals
-
※2PIC/S: A combination of abbreviations for the Pharmaceutical Inspection Convention (PIC) and the Pharmaceutical Inspection Co-operation Scheme (PICS), which are two international cooperation organizations that aim to improve cooperation among governments and inspection authorities in the areas of GMP and GDP
-
※3GDP (Good Distribution Practice): Quality management guidelines for the transportation and storage of pharmaceuticals
Expanding services into the fields of rare diseases and cell and gene therapy (CGT)
In 2012, we became the first pharmaceutical wholesaler to offer comprehensive support services in the field of rare diseases. Using our healthcare distribution platform, we were able to flexibly respond to needs that could not be served by existing distribution systems and made the reliable supply of orphan drugs a reality.
In 2016, we added to these efforts by initiating the distribution of clinical trial products for cell and gene therapy (CGT). For these products, there are cases in which there is a need to collect tissue or cells in Japan, ship them overseas, where they are processed or cultivated, and then return them to Japan. To serve this need, we collaborated in 2018 with World Courier, a part of the U.S.-based Cencora (former AmerisourceBergen) drug wholesale group, to create a global distribution platform for CGT products
In promoting the Cubixx System, we are making quality-preserving temperature-controlled logistics and total traceability a reality.
The Cubixx System, which we began deploying in 2017 as a distribution model for shipping specialty drugs from wholesale distribution facilities to medical institutions, applies IoT and RFID※4 digital technologies through collaborations with various other companies to make total traceability a reality. Offering visual representations of temperature, physical disturbance, location, and other data, the system provides 24/7 real-time monitoring of pharmaceutical transit and storage conditions, enabling resale and inventory adjustment decision-making. The Cubixx System contributes greatly to the reduction of pharmaceutical disposal losses at medical institutions and pharmacies. In 2021, we introduced new Cubixx System versions for home use, large-capacity needs, and room temperature storage, adding to the existing product line of systems for hospitals, pharmacies, and clinical trials. The Cubixx System is currently in use at over 400 facilities, mainly core hospitals nationwide.
-
※4RFID (Radio Frequency Identification): Automatic recognition system that uses radio signals to read and write stored information about individual people or objects

Start of delivering drugs to patients' homes using VIXELL cooling box for constant-temperature transportation of pharmaceuticals
We have developed a last-mile delivery system to patients' homes using the VIXELL cooling box for constant-temperature transportation of pharmaceuticals developed jointly with Panasonic, and built a total traceability distribution model from pharmaceutical companies to patients' homes.
VIXELL uses a cooling box and a thermal storage unit to prevent cold air from leaking through gaps, and can monitor temperature, location information, and impacts in real time with adopting a vacuum insulation housing as its material.
In 2022, we launched a residence delivery service using VIXELL for the therapeutic agent for hemophilia, Byclot Combination Intravenous Injection. Collection from medical institutions and delivery to patients' homes are handled by delivery drivers who received education specialized in drug delivery by GENie, which operates the ARUU prescription drug immediate delivery service by the Seino Group, a partner company.
Responding to the needs of foreign companies newly entering Japan’s pharmaceutical market
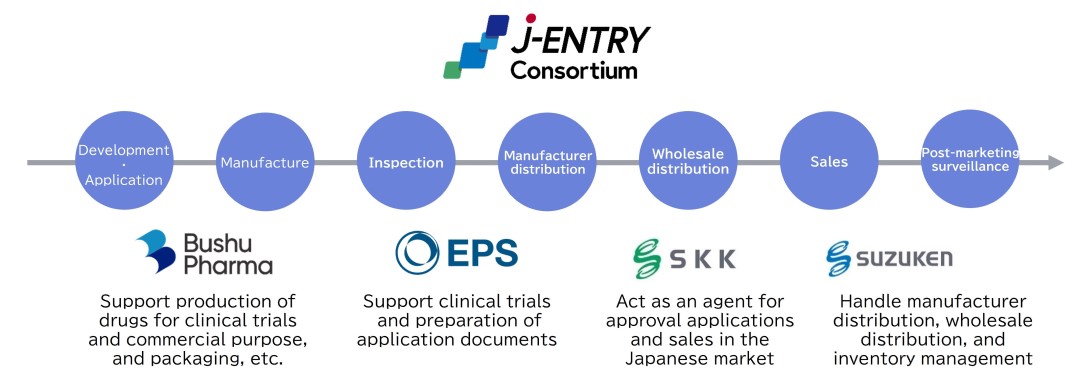
In recent years, the number of foreign pharmaceutical companies aiming to enter the Japanese market has been increasing, but many have no manufacturing or distribution centers, or distribution networks.
The Suzuken Group entered into a collaboration with Bushu Pharmaceuticals Ltd., in April 2021 and launched operations at the Greater Tokyo Distribution Center in April 2024. In addition to a wholesale distribution area, this Center also features an on-site contract manufacturing area and a manufacturer distribution area. Bushu Pharmaceuticals’ Soka Packaging Center is located within the contract manufacturing operations area, enabling on-site contract manufacturing that focuses on the inspection, labeling, and packaging of pharmaceutical products, as well as storage services. By offering a one-stop solution from manufacturing to wholesale distribution at a single site, we have minimized product movement, resulting in reduced lead times for deliveries, reduced transportation costs, and reduced environmental impact.
In May 2024, through our partnership with Bushu Pharmaceuticals and EPS Holdings, we established a new business model, the "J-ENTRY Consortium", a new business model, that contributes to eliminating Japan’s drug loss by enabling one-stop support for overseas pharmaceutical companies entering the Japanese market. This support covers all stages, from the development of specialty drugs to application for marketing approval, manufacturing, sales and marketing, and distribution. This business model operates on a contingency fee basis, with development fees and other necessary cost for placing the drugs on the market in Japan collected after their successful market launch, thereby allowing pharmaceutical companies to enter the market while minimizing their financial risk. By dealing with Japan-specific regulations and resolving issues such as the lack of a manufacturing facility, distribution center, or distribution network in Japan, this model will increase the number of companies entering the market, thereby contributing to expanding treatment options in Japan.
Related contents
- Business Overview: Pharmaceutical Distribution Business
- Business Overview: Healthcare-Related Services Business
The transformation to a health creation enterprise
Go to the top of “Transformation to a health creation enterprise”
